

Lac teol fort sachets
Model: P6MP0101
Product Description
Lac teol fort sachets
Presentation
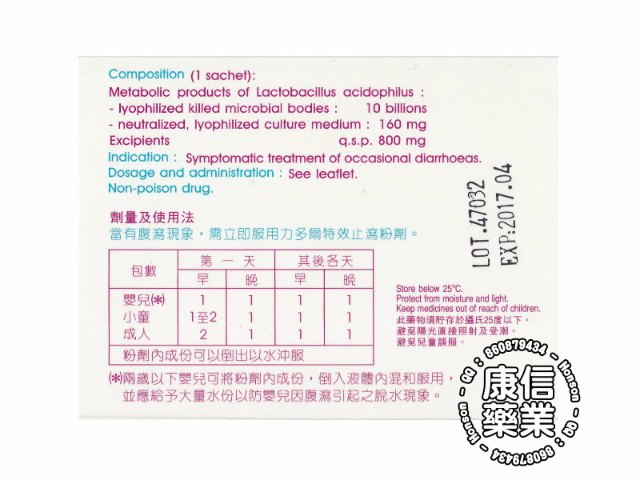
Oral powder in sachet. Boxes of 6, 10 and 100 sachets. Composition (1 sachet)
Metabolic products of Lactobacillus acidophilus :
.Lyophilized killed microbial bodies :.................10 billions
.Neutralized and lyophilized culture medium :.......160 mg
Excipients : Lactose monohydrate, calcium carbonate, silicic acid, banana-orange flavour, saccharose q.s.p......... 800 mg
Pharmacological properties Antidiarrheal of microbial origin, with :
.direct bacteriostatic action, and stimulation of the growth of the defensive acidogenic intestinal flora,
.non - specific immunostimulation of the mucosa (increases synthesis of IgA),
Indications
Symptomatic treatment of occasional diarrheas.
Disposal of product
Discard the sachets after the expiry date and throw contents into the dustbin.
Stability and storage
Read expiry date on the package - Store below 25°C
Dosage and administration
To be taken at the first sign of diarrhea
Infants () : Frist day -1st dose 1, 2nd dose 1, following day-1st dose 1 package, 2nd dose,
Children : Frist day -1st dose 1 to 2, 2nd dose 1, following day-1st dose 1 package, 2nd dose,
Adults : Frist day -1st dose 2, 2nd dose 1, following day-1st dose 1 package, 2nd dose,
Contents of sachets can be mixed into water
(•) Infants (< 2 years) ; To be used in conjunction with rehydration therapy. Pour out the content into water.
Usage during pregnancy
No known teratogenic effects have been reported.
Adverse side effects
No side effect has been so far reported.
Incompatibilities and interactions
No known incompatibilities and drugs interactions have been reported.
Non-posion drug